Environment & Energy
Related: About this forumScientists make mind-blowing breakthrough while studying energy transfer in seawater: 'We could create a circularity'

Scientists make mind-blowing breakthrough while studying energy transfer in seawater: 'We could create a circularity'
TheCooldown.com | Ren Venkatesh | April 27, 2025
Researchers at Northwestern University recently set out to extract solid minerals from seawater for concrete reinforcement. By splitting water molecules to draw out the mineral precipitate, the team hoped to produce the sand-like or gravel-like material required for concrete development more sustainably.
The university report described the result as a "carbon-negative building material," meaning the team's mineral output removed more carbon dioxide from the atmosphere than the process emitted.
As our global temperatures continue to rise as a result of the heat trapped by carbon pollution, discovering ways to capture the carbon dioxide already present in our atmosphere may prove just as important as reducing these emissions.
Revolutionizing the building industry is a huge step in the right direction. Cement production accounts for 8% of global carbon pollution, according to the World Economic Forum. "Depending on the ratio of minerals, the material can hold over half its weight in CO2," added the university report.
While using renewable energy to split water molecules is nothing new — scientists have looked to seawater for "green hydrogen" extraction for years, noted the Advanced Science News journal — the Northwestern team was the first to study the mineral residue from splitting seawater...more
https://www.thecooldown.com/green-tech/splitting-seawater-concrete-carbon-negative/
Goonch
(4,100 posts)
LiberalArkie
(19,084 posts)erronis
(21,843 posts)Color me skeptical.
Bernardo de La Paz
(60,153 posts)NNadir
(36,850 posts)The reality is that we are using more carbon intensive energy than at any time in history.
The confusion between the verb "to be" in its forms is and are and the conditional auxiliiary verb "could" is having tragic results.
Things are getting worse faster than ever not better.
The hydrogen hypers are here to sell fossil fuels by pretending that wasting energy to make hydrogen is "green." It isn't.
xocetaceans
(4,303 posts)It is an open-access paper, so that is good.
Nishu Devi, Xiaohui Gong, Daiki Shoji, Amy Wagner, Alexandre Guerini, Davide Zampini, Jeffrey Lopez,
Alessandro F. Rotta Loria
First published: 18 March 2025 | https://doi.org/10.1002/adsu.202400943
Abstract
Seawater offers immense potential for addressing global energy and climate challenges. Electrochemical seawater splitting is a sustainable approach for hydrogen production and carbon dioxide (CO2) sequestration, producing hydrogen gas at the cathode and oxygen or chlorine gas at the anode. Simultaneously, minerals such as calcium carbonate and magnesium hydroxide precipitate at the cathode, especially when coupled with CO2 injections for the sake of CO2 sequestration. These precipitates are often dismissed as energy-intensive byproducts. However, they have untapped potential as resources for construction, manufacturing, and environmental remediation. Here, a comprehensive experimental investigation is presented into the electrochemical precipitation of minerals in seawater under varying operational conditions. By systematically varying applied voltage, current density, and CO2 flow rate, the conditions that optimize mineral yield and selectivity while minimizing energy consumption are revealed. The findings advance the understanding of electrochemical synthesis and material processing in aqueous solutions, with a particular focus on the mineralization of calcareous compounds and their transformation into large-scale aggregates. These findings also support an additional and highly scalable application of seawater electrolysis, encompassing not only oceanic renewable hydrogen production and CO2 sequestration but also the sustainable production of carbon-trapping minerals and aggregates.
1 Introduction
Ocean and sea waters are a precious resource for human development and the Earth's ecosystem, covering more than 70% of the planet's surface[1] and representing ≈97% of the available water reserves.[2] Preserving and sustainably managing ocean and sea waters is essential to maintain their functions and ensure a balanced, livable planet.
...
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adsu.202400943
Here is a link to Northwestern's advertisement of the research:
erronis
(21,843 posts)Tomorrow (but never today.)
NNadir
(36,850 posts)...despite squandering trillions upon trillions upon trillions of dollars, it isn't even close to doing.
Like the hydrogen scam, so called "renewable energy" is nothing more than lipstick on the fossil fuel pig, a trivial and expensive reactionary fantasy that has left the planet in flames.
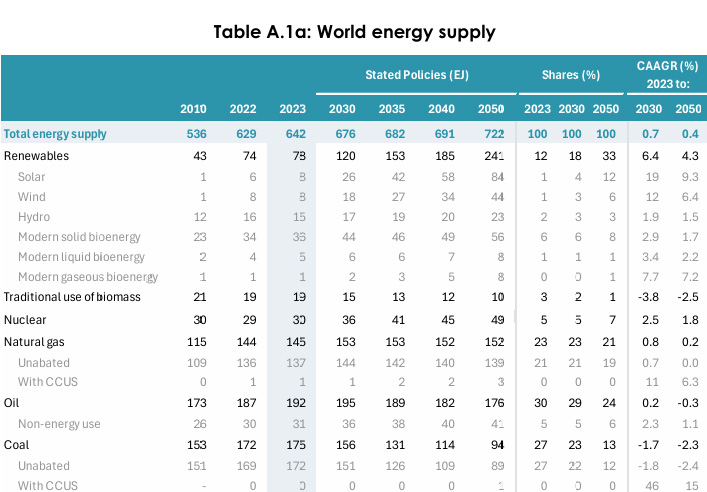
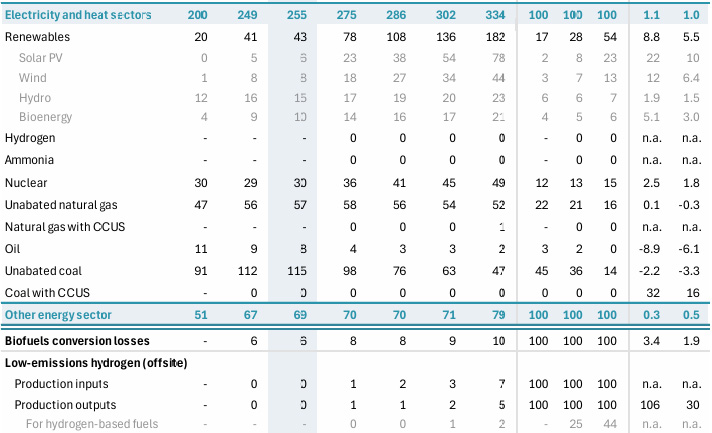
IEA World Energy Outlook 2024
Table A.1a: World energy supply Page 296.
I have no use for wishful thinking university press releases, if one must know.