Revealing the secrets to good catalytic performance in metal sulfides
Study provides catalyst design guidelines for hydrogen production through water electrolysis
July 23, 2025
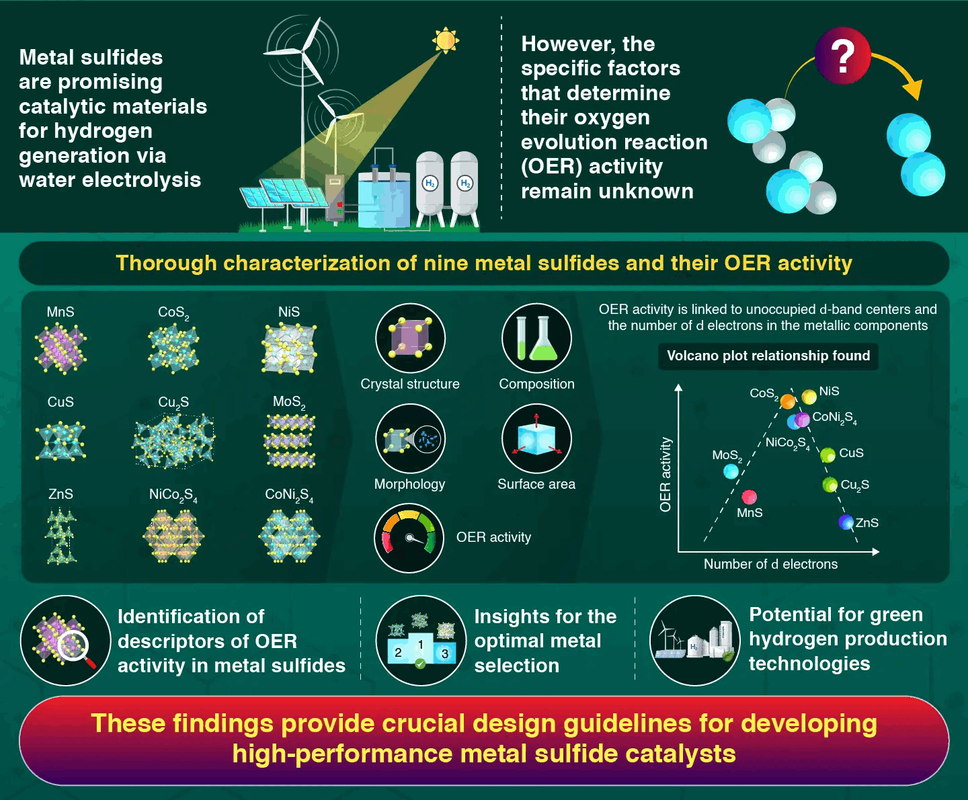
Metal sulfides with seven to eight d electrons show optimal performance as catalysts for water electrolysis, as reported by researchers from Institute of Science Tokyo. In a comprehensive analysis of various metal sulfides, they identified a volcano-shaped relationship between catalytic activity and the number of d electrons in metal atoms. This newly uncovered principle will form the basis of catalyst design guidelines, accelerating the development of efficient water-splitting catalysts for green hydrogen production
 Electrochemical Oxygen Evolution Catalysis of Metal Sulfides: A Systematic Study of Electronic Effects
Electrochemical Oxygen Evolution Catalysis of Metal Sulfides: A Systematic Study of Electronic Effects
Sugawara et al. (2025) |
Catalysis Science & Technology
The global energy crisis and the urgent need to combat climate change have made the development of green hydrogen a top priority. As a fuel that boasts exceptional energy density and produces only water when used, hydrogen is a highly attractive alternative for fossil fuels. Moreover, hydrogen can be obtained by splitting water molecules through a process known as water electrolysis, which in turn can be driven by electricity derived from renewable sources. However, a significant hurdle remains: the oxygen evolution reaction (OER), which occurs at the anode in the presence of precious metal-based catalysts during water electrolysis, still represents a major bottleneck.
…
The team’s analysis revealed two key factors. First, they found that metal sulfides with lower-energy unoccupied
d-orbital centers in their metal atoms showed superior catalytic activity—a relationship similar to what had been observed in metal oxides but never before demonstrated for sulfides. More remarkably, they discovered that catalytic activity forms a volcano-shaped graph when plotted against the number of
d electrons in the metal atoms, peaking between seven and eight d electrons. This volcano plot relationship occurs because metals with fewer
d electrons bind reaction intermediates too strongly, while those with too many bind them too weakly. Thus, optimal performance appears to require a balanced interaction. “For the first time in the world, we have discovered a previously unknown principle—that the activity of metal sulfides is determined by the number of
d electrons in the metal,” highlights Yamaguchi.
Taken together, these findings provide researchers with concrete guidelines for designing more effective catalysts. By using
d electrons as performance descriptors in metal sulfide candidates, scientists can now predict which combinations are most likely to function as high-performance OER catalysts, thereby reducing development time and costs.
…
