Issues in Water Chlorination Toxicology: ID of High Molecular Weight Halogenated Molecules in Drinking Water [View all]
The paper to which I'll refer in this post is this one: Unravelling High-Molecular-Weight DBP Toxicity Drivers in Chlorinated and Chloraminated Drinking Water: Effect-Directed Analysis of Molecular Weight Fractions Huiyu Dong, Amy A. Cuthbertson, Michael J. Plewa, Chad R. Weisbrod, Amy M. McKenna, and Susan D. Richardson Environmental Science & Technology 2023 57 (47), 18788-18800.
In my personal professional work, I am often tasked, although it's hardly my main focus, with the peripheral task of understanding environmental contaminants found environmental matrices as they appear in human blood, skin, and other biological matrices. This was a big concern during the Covid crisis, particularly with the components of hand sanitizers subject to percutaneous absorption.
One thing is very clear these days if one regularly reads the environmental literature, as I do: The breadth and number of environmental contaminants in water, land, and indeed the air, is rising very fast.
The chlorination of water - it's disinfection - has saved many tens of millions of lives, perhaps hundreds of millions of lives and prevented the disease related disability even more widely - but like any technology, it introduces its own set of risks; one must carefully weigh risk benefit analysis with any technology. One also would be well advised to reconsider any widely used technology based on environmental changes which may change that risk/benefit analysis.
This article is a reflection of this type of analysis, and I would add, involves some very sophisticated science with which I am enamored, high resolution mass spectrometry.
From the article's introduction:
As chloramination forms lower levels of regulated THMs and HAAs than chlorination, chloramine has been increasingly adopted as a secondary disinfectant to maintain a residual disinfection ability in distribution systems. (10−12) However, TOX formed during chloramination (generally less than that from chlorination) can still reach considerable levels, depending on the chloramine dose, Cl/N ratio, pH, and other conditions. (13) Moreover, in chloraminated drinking water, up to 70% of TOX formed remains unknown, a significantly higher unknown fraction than observed for chlorination (∼50% of TOX unknown). (14,15) The unknown TOX formed during chlorination and chloramination may contain a substantial amount of toxic DBPs, which need identification. (16,17)
Continued efforts have been made to identify unknown DBPs in drinking water, especially for the toxicity drivers. (18−28) The identification of unknown DBPs and corresponding toxicity assessments significantly enriched the knowledge of unknown TOX. One of the tricky challenges in the assessment of complex drinking water mixtures is the identification of those DBPs that contribute significantly to observed toxicity. A recent forcing factor study of U.S. drinking waters, which combined the quantification of 72 DBPs with whole-water cytotoxicity measurements, found unregulated dihaloacetonitriles to be the main cytotoxicity drivers in drinking water, along with iodo acids in chloraminated drinking waters containing iodide. (29) When measured whole-water toxicity data are not available, another approach called “TIC-Tox” is often used, where each measured low-molecular-weight DBP is multiplied by the reported cytotoxicity or genotoxicity index of that DBP measured in Chinese hamster ovary (CHO) cells. (30) However, the TIC-Tox method is limited to the ∼100 low-molecular-weight known DBPs for which quantitative cellular toxicity data have been evaluated. For unknown/unquantified DBPs, it is difficult to use the TIC-Tox method to identify unknown DBP toxicity drivers. (7)...
The authors explain that while the analytical technology traditionally used for water analysis, GC/MS or GC/MS/MS (Gas Chromatography Mass Spectrometry, single quad and tandem respectively), these are really only suitable for volatile compounds. The authors chose to use a related technology, LC/MSn, where n refers to high resolution mass spectrometry which can pick up heavier molecular weight compounds. In fact they use the highest resolution for of mass spectrometry commercially available, FT-ICR-MS, Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. (In my dreams...) In fact they use the mass spectrometer with the highest resolution in the world, the 21 Tesla FT-ICR-MS at the National Magnetic Laboratory located in neofascist Floridastan. It has a mass resolution of over 1 million ppm. (Ron DeSantis has yet to completely kill science in his Taliban inspired state, inasmuch as the facility is funded by the United States Government, from which Desantis probably wants, along with his fellow racists, to secede, and not his provincial anti-science zealots in his government.) This mass resolution, giving five significant figures after the decimal point, is sufficient to clearly delineate the number of chlorine atoms in a molecule based on the isotopic distribution of chlorine's two isotopes, 35Cl having an exact mass of 34.96885269 amu, and 37Cl having an exact mass of 36.96590259 amu.
A figure in the paper describes this procedure for a sample contaminant:
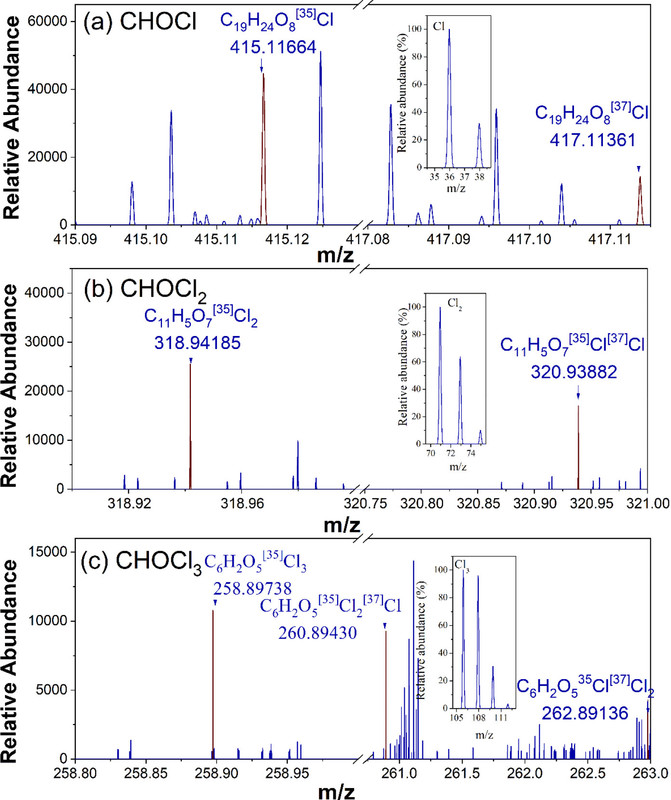
The caption:
The distribution of types of halogenated contaminants.
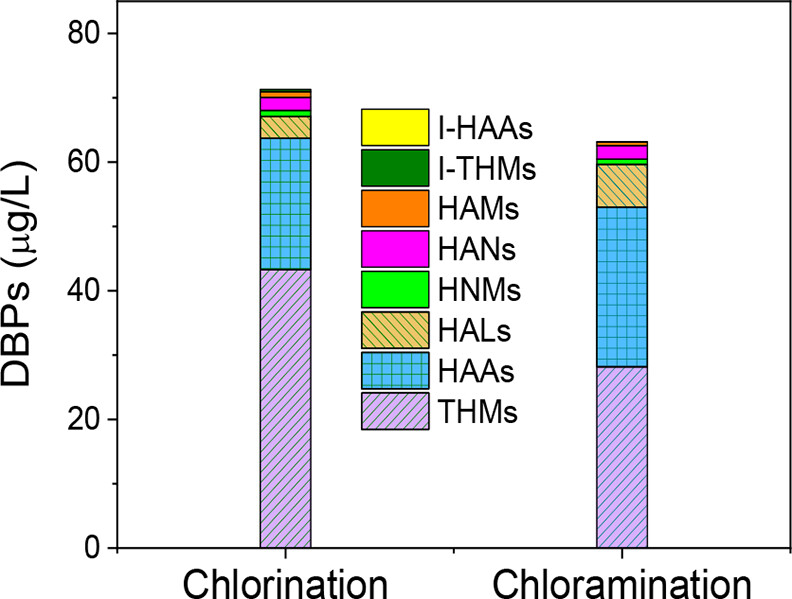
The caption:
The abbreviations in the graphic are described in the text:
A type of analysis using 2D plots of atomic ratios in molecules (Van Krevelen plots) is utilized to give a general overview of the types of molecules found among the halogenated species:
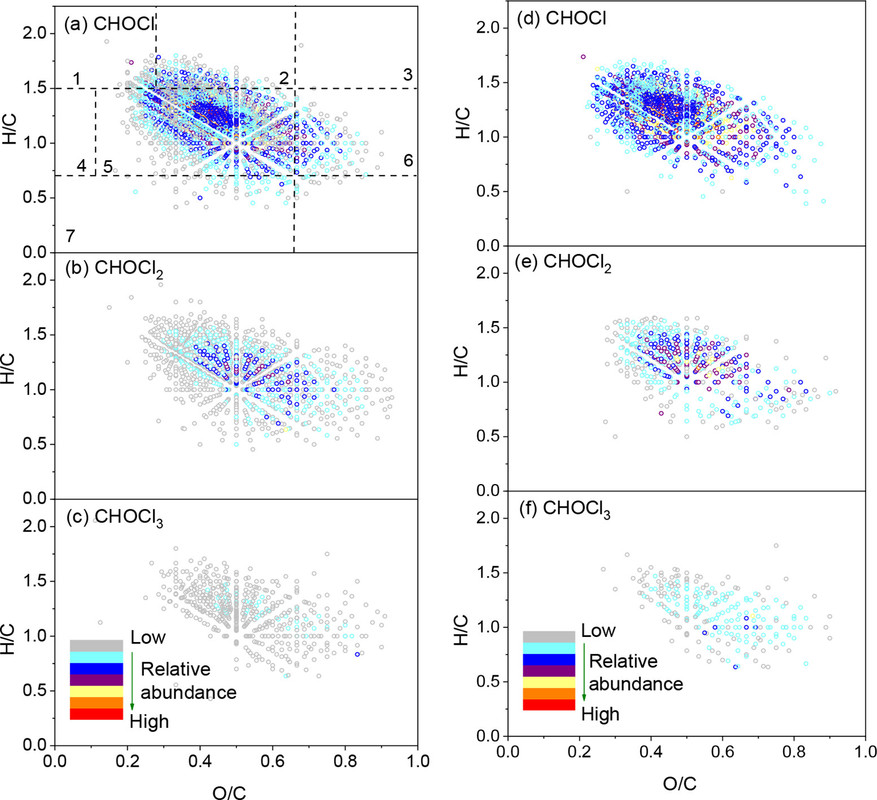
The caption:
The paper also includes studies of the toxicology of sample compounds.
I trust you're having a pleasant weekend.