...as a nuclear fuel, a very high neutron multiplicity.
The most commonly available isotope is 241Am, the decay product of 241Pu (t1/2 = 14.290 years).
It is definitely accumulating in used nuclear fuels, and, in fact, unused isolated reactor grade plutonium, like the significant stockpile in the UK.
The neutron multiplicity (aka "nubar" ) is shown in this graph:
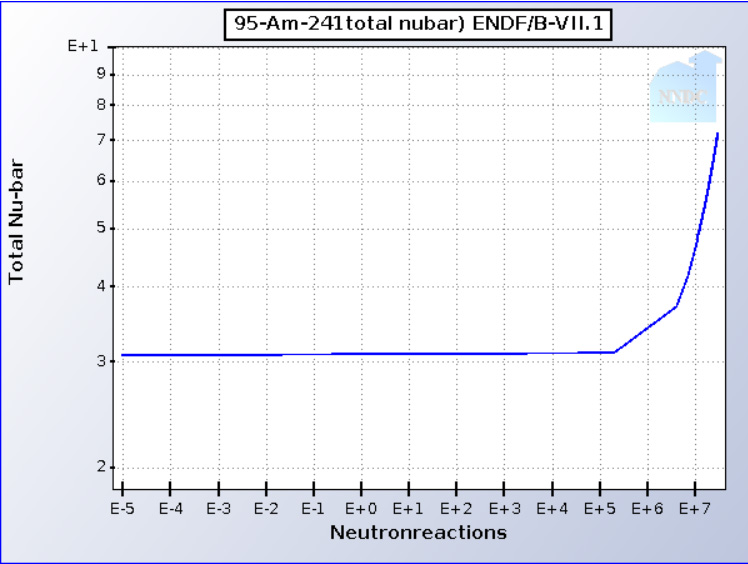
BNL Nuclear Data
241Am is not really fissionable in thermal reactors, as the capture to fission ratio is too high but it can reach a critical mass in the fast neutron spectrum. Critical masses of the three accessible Americium Isotopes. In cases where capture occurs, it creates the highly fissionable 242Am and 242mAm nuclear isomers, the latter of which can accumulate in considerable fractions, owing to its long half-life, (t1/2 = 141 years). It's multiplicity in the fast spectrum approaches 4.
It takes three neutron captures to generate 241Am. (Interestingly the precursor, 241Pu, also has a very high neutron multiplicity in the epithermal region). Thus all of the neutrons that went into making it can be recovered, and at one MeV or more (fissioning actinides general emerge at 1 - 2 MeV before being thermalized by a moderator).
This makes americium a very attractive fuel to my mind. My son's girlfriend, also a nuclear engineering Ph.D. student, reports that americium is her favorite element, which is just one of the reasons I like her very much. I have a cool idea about an americium fueled high temperature reactor, and if I can't get my son to listen to it, perhaps she will.
It's certainly available now in multiton quantities, albeit in dilute solid solutions. I think it should be readily concentrated in fluoride volatility reprocessing schemes.
